H Is An Acid According To
H Is An Acid According To. Acids can be classified according to its strength ( degree of ionization ) , Its source and Basicity , Acids Both of citric acid and phosphoric acid have the same degree of basicity, while they differ in their source ( origin), Because both of them are tribasic acids , but citric acid is an organic acid, while. Learn vocabulary, terms and more with flashcards, games and other study tools.
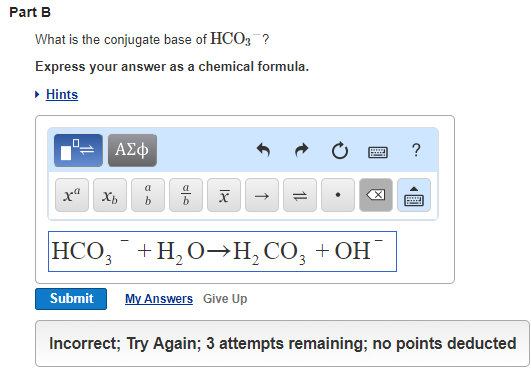
Strong Acid: Strong acids release all The strength of an acid is determined by the polarity and the atomic sizes of the acid molecule.
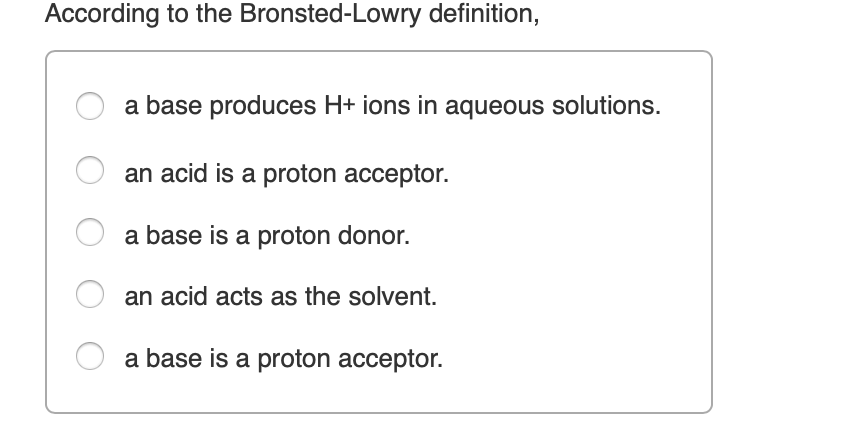
Where A+is an electron acceptor or Lewis acid whereas Bˉ is an electron donor or Lewis base, and A-B is a coordinate covalent compound.
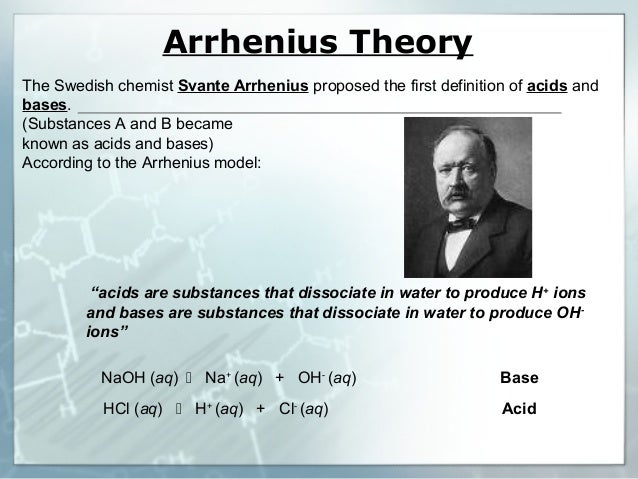
Acids can be classified according to its strength ( degree of ionization ) , Its source and Basicity , Acids Both of citric acid and phosphoric acid have the same degree of basicity, while they differ in their source ( origin), Because both of them are tribasic acids , but citric acid is an organic acid, while. Sulfuric acid is a strong dehydrating substance, and concentrated sulfuric acid forces water out of various compounds. An acid dissociation constant, Ka, (also known as acidity constant, or acid-ionization constant) is a quantitative measure of the where HA is a generic acid that dissociates by splitting into A−, known as the conjugate base of the acid, and the hydrogen ion or proton, H+, which, in the case of aqueous.



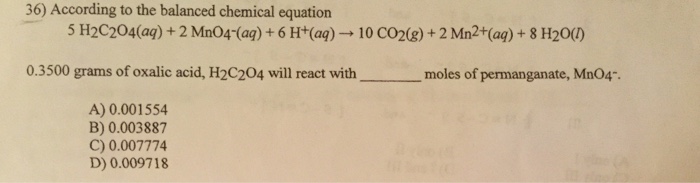

0 Response to "H Is An Acid According To"
Posting Komentar