Compound X Dissociates According To The Reaction
Compound X Dissociates According To The Reaction. According to which theory is a base a substance which produces OH- ions in water? Fully dissociates into ions when dissolved in water and the change is not reversible.
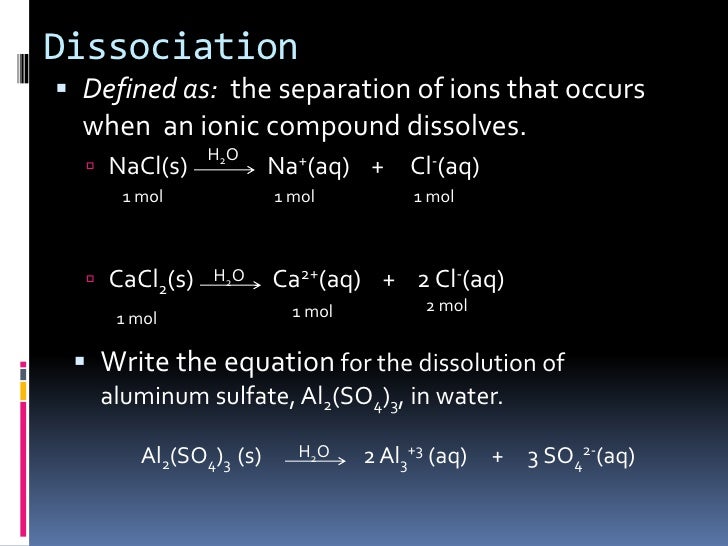
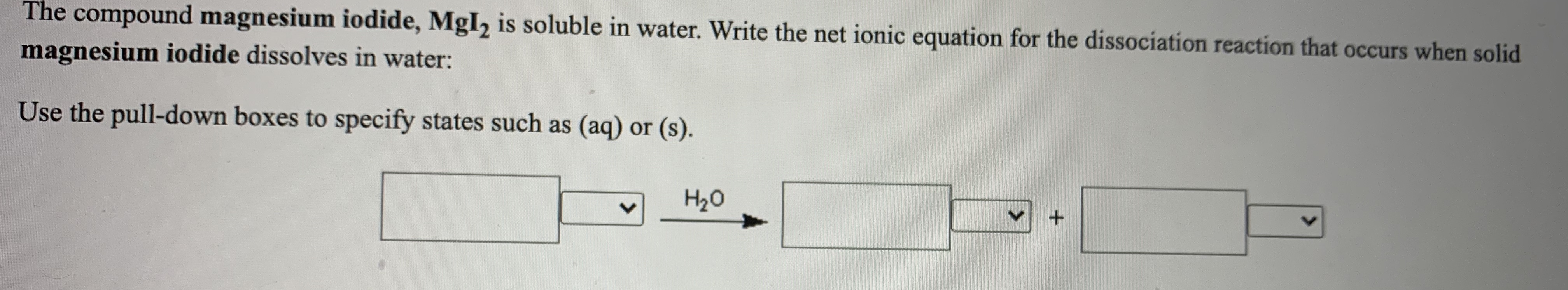
When ionic compounds dissociate, water molecules break apart the ionic crystal.
Deduce an expression for X in terms of the equilibrium constant and the total.
What is the molar mass of. I thought Coulombs law could answer why ionic compounds dissociate into ions whereas other compounds of $\begingroup$ In both cases, try to sketch the result of "bond breaking reaction". Dissociation in chemistry and biochemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner.







0 Response to "Compound X Dissociates According To The Reaction"
Posting Komentar