According To 1st Law Of Thermodynamics
According To 1st Law Of Thermodynamics. Bicycle pump is the first law of thermodynamics example. The law says that this total amount of energy is constant.
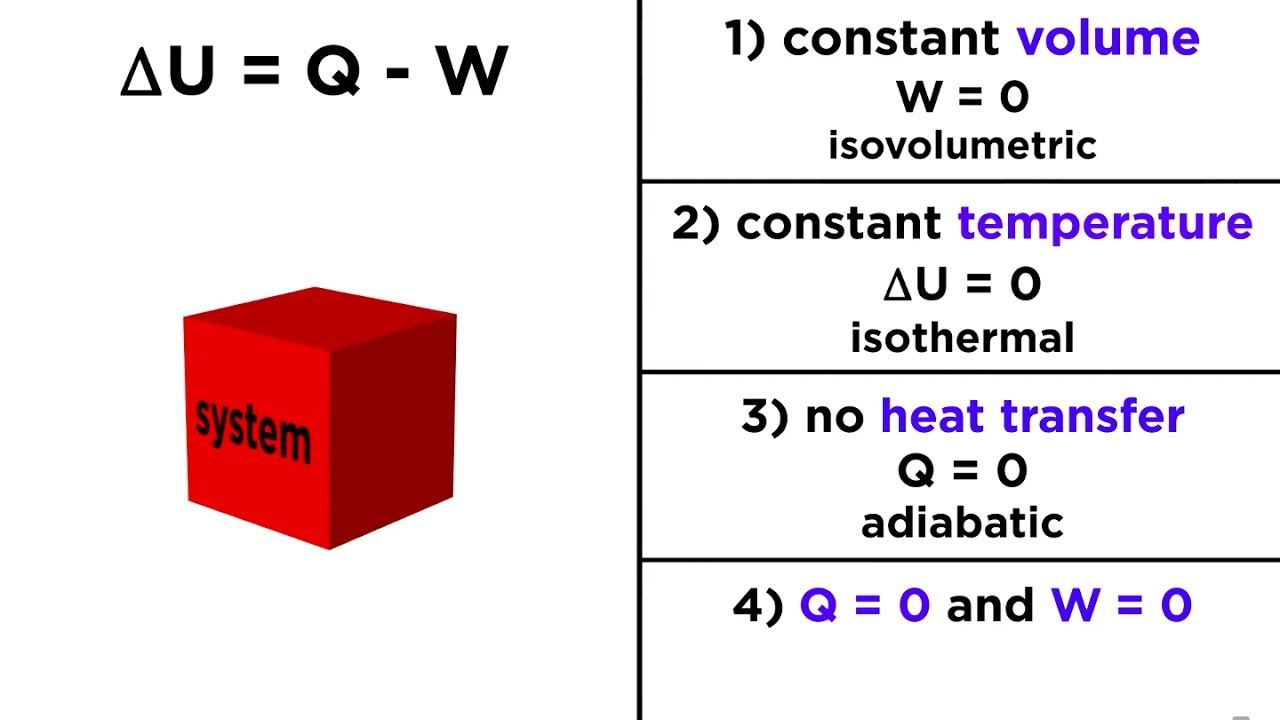
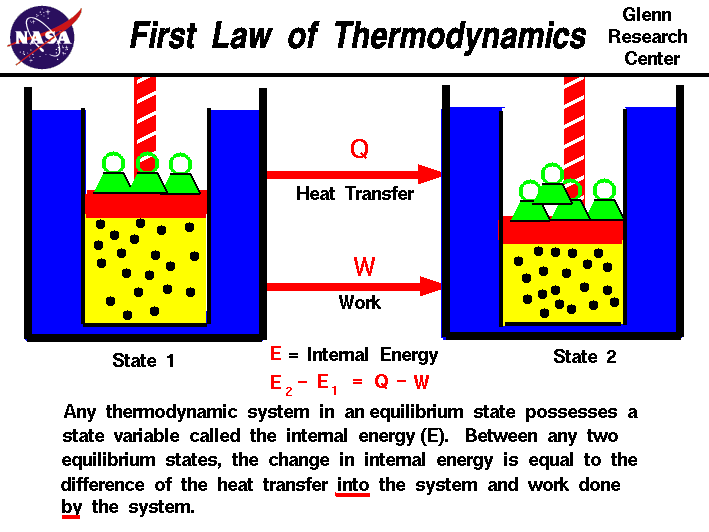
The First Law of Thermodynamics states that the increase in internal energy of a closed system equals the amount of heat energy added minus the work performed by the system.
The first law of thermodynamics states that; for any spontaneous or non spontaneous reaction the sum of energy of the system and its surrounding remain constant.
Therefore, they will be discussed first, to form a sound basis for the development of. Like every other SCIENTIFIC statement/hypothesis/theory, it has to be FALSIFIABLE. The first law is a conservation law (Law of Conservation of Energy).







0 Response to "According To 1st Law Of Thermodynamics"
Posting Komentar