Q According To Avogadros Hypothesis
Q According To Avogadros Hypothesis. Avogadro's hypothesis states that equal volumes of all gases at the same temperature and pressure contain equal numbers of particles. The law is a specific case of the ideal gas law.
Avogadro's hypothesis states that equal volumes of all gases at the same temperature and pressure contain equal numbers of particles.
Yes, according to Alejandro, have notices, if all gases have equal volumes.
The reaction between Hydrogen and Oxygen according to Avogadro is shown in the following diagram. Question is according to robert Rose hypothesis, it will volumes of all gases under the same conditions of temperature and pressure will contain same number of molecules. Let us consider above reaction and apply Avogadro's hypothesis.
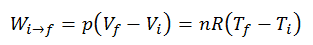




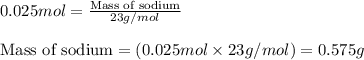
0 Response to "Q According To Avogadros Hypothesis"
Posting Komentar